By TRUNNANO | 04 January 2021 | 0 Comments
How does sodium stearate play its role in daily life
What is sodium stearate? What is the chemical formula of sodium stearate?
Sodium stearate usually refers to sodium dodecanoate. The chemical structure of sodium stearate is Na (C18H35O2) {that is (C17H35COO) Na}, and the molecular weight is 306.46. Melting point: 250°C~270°C, white powder or white lumps, creamy, fatty, easily soluble in hot water or alcoholic water, absorb moisture in the air, the solution becomes alkaline due to hydrolysis.
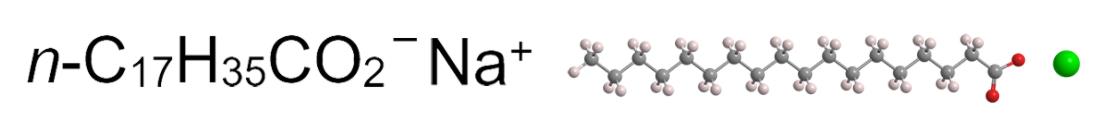
What is sodium stearate used for?
Main role
1. Detergent: used to control foam during rinsing (sodium stearate is the main component of soap).
2. Emulsifier or dispersant: used for polymer emulsification and antioxidant.
3. Corrosion inhibitor: has a protective effect on polyethylene packaging film.
4. Cosmetics: shaving gel, transparent viscose, etc.
5. Glue: Use as a natural glue and then paste paper
In addition to being the main soap ingredient, sodium stearate is also used as an additive in other cosmetics to form a solid "stick-like" shape. Sodium stearate has many other uses, including emulsifier and dispersant in latex paint; ink thickener.
Stabilizer, thickener and dispersant for liquid cosmetics; FDA approved flavor additives; viscosity modifier in gel perfume; lubricant in polycarbonate and nylon; lubricant and dust remover in rubber production.
In addition, sodium stearate can also be used as a heat stabilizer for polyethylene. It has excellent lubricity and good processing properties. It has a synergistic effect when used with zinc soap and epoxy compounds, which can improve thermal stability. When used with lead salt and lead soap in hard products, it can increase the speed of gelatinization.
Sodium stearate can also be used in polyethylene and polypropylene to eliminate the adverse effects of residual catalyst on the color and stability of the resin. It is also widely used as a lubricant and release agent for thermosetting plastics such as polyolefins, polyester reinforced plastics, and phenolic amino resins.
Sodium stearate soap formula
About five thousand years ago, humans began to use detergents similar to modern soaps. Early rough soaps were made with natural oils and available alkaline materials (such as wood ash). During the industrial revolution, manufacturers began to use pure fatty acids and alkalis (such as lye (sodium hydroxide or potassium hydroxide), quicklime (calcium oxide) or slaked lime (calcium hydroxide)) to make more refined soaps.
Sodium stearate is the most common fatty acid salt in soaps today. Common sources of stearic acid raw materials are vegetable triglycerides obtained from coconut oil and palm oil, and animal triglycerides obtained from tallow. The names of stearic acid and stearic acid are derived from the Greek word "tallow" stéar.

First, we need to determine the most important ingredient formula for making soap:
1. Oil
Oil is the main raw material for the saponification reaction. As long as you use vegetable oil/animal oil to make soap, you can usually use a variety of practical oils, but olive oil (no taste) is recommended. Animal oil usually uses lard because it is easily available and inexpensive.
2. Alkali (sodium hydroxide)
Alkaline water is an aqueous solution of sodium bicarbonate, mainly used as a catalyst for the saponification reaction, and reacts with oil to form sodium stearate, which is the soap we use.
3. Water
It is a carrier that does not participate in any reaction and is mainly used as a reaction carrier for the saponification reaction.
What is the process of making simple soap?
1. First make alkaline water, dissolve it with sodium hydroxide, and then stir to form alkaline water.
2. Put the oil and alkali into a fast glass/ceramic container and carry out a full saponification reaction.
3. Take out the solid sodium stearate, the product of the saponification reaction, and remove the remaining reactants from other reactions.
4. Put the solid in the mold until it is dry, and turn it into soap after releasing the mold.
Because sodium hydroxide solution is strongly alkaline and corrosive, care needs to be taken during preparation.
Is sodium stearate good for the skin?
Compared with some other surfactants, sodium stearate is actually considered mild, which means it is less likely to irritate the skin. Stearic acid has no obvious damage to the skin because stearic acid is a common fatty acid that is widely present in various oils. The content of animal fat is high, especially the highest content in butter, while the content of vegetable fat is low but it also exists. Stearic acid can be synthesized into stearic acid compounds and is widely used in cosmetics or lubricants and other chemical raw materials. The use of cosmetics mainly plays a role in lubrication and emulsification. Therefore, many cosmetics contain other stearic acid compounds, as long as their content is within the standard range, they will not cause obvious damage and irritation to the skin.

Is sodium stearate the same as baking soda?
Sodium bicarbonate is called baking soda. Sodium stearate is just a saponified stearic acid, whether it comes from tallow, stearic acid or kokum butter. Used as a thickener/gelling agent and co-emulsifier. This white solid is the most common soap.
Is sodium stearate natural? Why can sodium stearate remove oil?
Sodium stearate is a vegetable soap material derived from coconut oil and palm oil. It is commonly called sodium salt and is derived from stearic acid (a naturally occurring fatty acid).
Sodium stearate is a kind of salt and is a strong electrolyte. But stearic acid has a very large number of carbon atoms, and organic matter with a large number of carbon atoms is insoluble in water, but soluble in organic matter, that is, oil. Although sodium ions are ionized, the hydrophilic ends of the remaining stearate groups still exist.
Sodium stearate usually refers to sodium dodecanoate. The chemical structure of sodium stearate is Na (C18H35O2) {that is (C17H35COO) Na}, and the molecular weight is 306.46. Melting point: 250°C~270°C, white powder or white lumps, creamy, fatty, easily soluble in hot water or alcoholic water, absorb moisture in the air, the solution becomes alkaline due to hydrolysis.
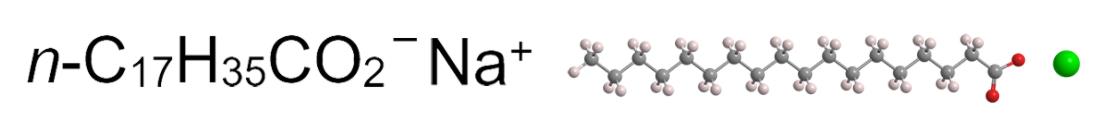
What is sodium stearate used for?
Main role
1. Detergent: used to control foam during rinsing (sodium stearate is the main component of soap).
2. Emulsifier or dispersant: used for polymer emulsification and antioxidant.
3. Corrosion inhibitor: has a protective effect on polyethylene packaging film.
4. Cosmetics: shaving gel, transparent viscose, etc.
5. Glue: Use as a natural glue and then paste paper
In addition to being the main soap ingredient, sodium stearate is also used as an additive in other cosmetics to form a solid "stick-like" shape. Sodium stearate has many other uses, including emulsifier and dispersant in latex paint; ink thickener.
Stabilizer, thickener and dispersant for liquid cosmetics; FDA approved flavor additives; viscosity modifier in gel perfume; lubricant in polycarbonate and nylon; lubricant and dust remover in rubber production.
In addition, sodium stearate can also be used as a heat stabilizer for polyethylene. It has excellent lubricity and good processing properties. It has a synergistic effect when used with zinc soap and epoxy compounds, which can improve thermal stability. When used with lead salt and lead soap in hard products, it can increase the speed of gelatinization.
Sodium stearate can also be used in polyethylene and polypropylene to eliminate the adverse effects of residual catalyst on the color and stability of the resin. It is also widely used as a lubricant and release agent for thermosetting plastics such as polyolefins, polyester reinforced plastics, and phenolic amino resins.
Sodium stearate soap formula
About five thousand years ago, humans began to use detergents similar to modern soaps. Early rough soaps were made with natural oils and available alkaline materials (such as wood ash). During the industrial revolution, manufacturers began to use pure fatty acids and alkalis (such as lye (sodium hydroxide or potassium hydroxide), quicklime (calcium oxide) or slaked lime (calcium hydroxide)) to make more refined soaps.
Sodium stearate is the most common fatty acid salt in soaps today. Common sources of stearic acid raw materials are vegetable triglycerides obtained from coconut oil and palm oil, and animal triglycerides obtained from tallow. The names of stearic acid and stearic acid are derived from the Greek word "tallow" stéar.

1. Oil
Oil is the main raw material for the saponification reaction. As long as you use vegetable oil/animal oil to make soap, you can usually use a variety of practical oils, but olive oil (no taste) is recommended. Animal oil usually uses lard because it is easily available and inexpensive.
2. Alkali (sodium hydroxide)
Alkaline water is an aqueous solution of sodium bicarbonate, mainly used as a catalyst for the saponification reaction, and reacts with oil to form sodium stearate, which is the soap we use.
3. Water
It is a carrier that does not participate in any reaction and is mainly used as a reaction carrier for the saponification reaction.
What is the process of making simple soap?
1. First make alkaline water, dissolve it with sodium hydroxide, and then stir to form alkaline water.
2. Put the oil and alkali into a fast glass/ceramic container and carry out a full saponification reaction.
3. Take out the solid sodium stearate, the product of the saponification reaction, and remove the remaining reactants from other reactions.
4. Put the solid in the mold until it is dry, and turn it into soap after releasing the mold.
Because sodium hydroxide solution is strongly alkaline and corrosive, care needs to be taken during preparation.
Is sodium stearate good for the skin?
Compared with some other surfactants, sodium stearate is actually considered mild, which means it is less likely to irritate the skin. Stearic acid has no obvious damage to the skin because stearic acid is a common fatty acid that is widely present in various oils. The content of animal fat is high, especially the highest content in butter, while the content of vegetable fat is low but it also exists. Stearic acid can be synthesized into stearic acid compounds and is widely used in cosmetics or lubricants and other chemical raw materials. The use of cosmetics mainly plays a role in lubrication and emulsification. Therefore, many cosmetics contain other stearic acid compounds, as long as their content is within the standard range, they will not cause obvious damage and irritation to the skin.

Is sodium stearate the same as baking soda?
Sodium bicarbonate is called baking soda. Sodium stearate is just a saponified stearic acid, whether it comes from tallow, stearic acid or kokum butter. Used as a thickener/gelling agent and co-emulsifier. This white solid is the most common soap.
Sodium stearate is a vegetable soap material derived from coconut oil and palm oil. It is commonly called sodium salt and is derived from stearic acid (a naturally occurring fatty acid).
Sodium stearate is a kind of salt and is a strong electrolyte. But stearic acid has a very large number of carbon atoms, and organic matter with a large number of carbon atoms is insoluble in water, but soluble in organic matter, that is, oil. Although sodium ions are ionized, the hydrophilic ends of the remaining stearate groups still exist.
Leave a Reply
Your email address will not be published.Required fields are marked. *
POPULAR BLOG
- What is graphene aerogel?
- What is Cadmium telluride CdTe and CdTe solar cell?
- Four types of surfactants and their differences and applications
- What is the explosive welding cladding of metal plates?
- What is EBS Ethylene Bis Stearamide?
- An article lets you understand the characteristics and applications of sodium silicate
- Advantages of graphite anode for lithium-ion battery
- What is Spherical Quartz Powder?
- What is sodium silicate?
- Characteristics and Application of Spherical Alumina
CATEGORIES
