By Dr.Qian from Guizhou University of Finance and Eco | 16 July 2020 | 0 Comments
The professional knowledge of graphite
What is graphite?
Graphite is an allotrope of the elemental carbon, and each carbon atom is surrounded by three other carbon atoms (arranged in a honeycomb of hexagons) that are covalently bonded to form a covalent molecule. Since each carbon atom emits an electron, those electrons can move freely, so graphite is a conductor of electricity. Graphite is one of the softest minerals, and its uses include making pencil leads and lubricants.  What is the definition of graphite powder?
What is the definition of graphite powder?
Graphite powder soft, black gray; Greasy and can contaminate paper. The hardness is 1 ~ 2 and increases to 3 ~ 5 along the vertical direction with the increase of impurity. Specific gravity is 1. 9 ~ 2. 3. Under the condition of starvation, it is one of the most heat-resistant mineral, whose melting point is above 3000 ℃. At room temperature, graphite powder has stable chemical properties and is insoluble in water, dilute acid, dilute alkali and organic solvents. Materials with high temperature conductivity, can do refractory materials, conductive materials, wear-resistant lubrication materials.  What is the chemical formula of graphite?
What is the chemical formula of graphite?
Graphite is an allotrope of carbon. It only consists of carbon atoms, so its formula or symbol is C. 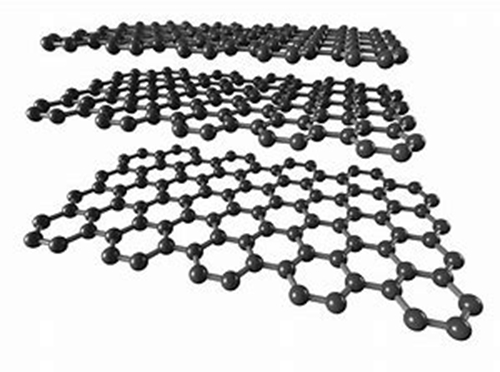 What are the properties of graphite?
What are the properties of graphite?
Graphite powder is a kind of highly reactive material, which will change its resistivity in according to its environment. But one thing will never change that is the graphite powder is one of the very good non-metallic conductive material. In the object of the insulation, as long as graphite powder is guaranteed uninterrupted, it also will be powered even as a thin line. However, the resistance value of graphite powder can not be told clearly, because the different degree of thickness of graphite powder will cause different applications in different materials and will bring the different resistivity in various environments. Due to its special structure, graphite has the following special properties:  1) High temperature resistant type: graphite melting point is 3850( + or - 50 ℃), and its boiling point is 4250 ℃. Even by the ultra high temperature arc ignition, weight loss is small, thermal expansion coefficient is small. Graphite with temperature increase and strengthen the intensity, at 2000 ℃, the intensity of graphite double.
1) High temperature resistant type: graphite melting point is 3850( + or - 50 ℃), and its boiling point is 4250 ℃. Even by the ultra high temperature arc ignition, weight loss is small, thermal expansion coefficient is small. Graphite with temperature increase and strengthen the intensity, at 2000 ℃, the intensity of graphite double.
2) Electrical and thermal conductivity: the electrical conductivity of graphite is 100 times higher than that of general non-metallic minerals. Its thermal conductivity is higher than steel, iron, lead and other metal materials. The thermal conductivity decreases with the increase of temperature. Even at extremely high temperature, graphite becomes an adiabatic.
3) Lubricity: the lubrication performance of graphite depends on the size of graphite scale. The larger the scale, the smaller the friction coefficient and the better the lubrication performance.
4) Chemical stability: graphite has good chemical stability at room temperature and can resist acid, alkali and corrosion of organic solvents.
5) Plasticity: graphite has good toughness and can be connected into very thin sheets.
6) Thermal shock resistance: when used at room temperature, graphite can withstand severe changes in temperature without damage. When the temperature changes abruptly, the volume of graphite does not change much and no cracks will occur.
What can the graphite be used for?  1, Graphite can be used as a refractory: graphite and its products with high temperature resistance, high strength properties, in the metallurgical industry is mainly used to manufacture graphite crucible. Besides, it is commonly used as a protective agent ingot, metallurgical furnace lining in steelmaking.
1, Graphite can be used as a refractory: graphite and its products with high temperature resistance, high strength properties, in the metallurgical industry is mainly used to manufacture graphite crucible. Besides, it is commonly used as a protective agent ingot, metallurgical furnace lining in steelmaking.
2, Graphite can be used as conductive materials: used in the electrical industry as a manufacturing electrode, brush, carbon rod, carbon tube, mercury positive current of the positive pole, graphite gasket, telephone parts, television picture tube coating.
3, Graphite can be used as wear-resisting lubrication material: graphite in the mechanical industry is often used as a lubricant. Lubricating oil can not be used in high speed, high temperature and high pressure conditions, and graphite wear-resisting material can be in (a) 200 ~ 2000 ℃ temperature under high sliding velocity, no lubricating oil. Many conveying corrosive medium equipment use graphite material made of piston cup, seal ring and bearing, and they do not need to add lubricating oil when running. Graphite emulsion is also a good lubricant for many metal processing (wire drawing, tube drawing).  Can the graphite pencil sketchings on a paper conduct electricity?
Can the graphite pencil sketchings on a paper conduct electricity?
We know that graphite leaves traces on paper only because of the friction between the graphite tip and the surface of the paper. This ensures that the surface of the paper is rough, otherwise the smooth surface of the paper will not allow the graphite molecules to hold their position on the paper, and the molecules will slide.
Due to the irregularity and roughness of graphite surface, the continuity of graphite molecules becomes uneven, there will be no proper flow of electrons, which will make electrical conduction almost impossible.
Because of the roughness of the surface, resistance rises rapidly, impeding the conduction of electricity, the result can be paper burning in high resistance areas.
These irregularities will cause the electrons to move from one point to another with a high potential. As a result, this can lead to paper burning.
Graphite is an allotrope of the elemental carbon, and each carbon atom is surrounded by three other carbon atoms (arranged in a honeycomb of hexagons) that are covalently bonded to form a covalent molecule. Since each carbon atom emits an electron, those electrons can move freely, so graphite is a conductor of electricity. Graphite is one of the softest minerals, and its uses include making pencil leads and lubricants.

Graphite powder soft, black gray; Greasy and can contaminate paper. The hardness is 1 ~ 2 and increases to 3 ~ 5 along the vertical direction with the increase of impurity. Specific gravity is 1. 9 ~ 2. 3. Under the condition of starvation, it is one of the most heat-resistant mineral, whose melting point is above 3000 ℃. At room temperature, graphite powder has stable chemical properties and is insoluble in water, dilute acid, dilute alkali and organic solvents. Materials with high temperature conductivity, can do refractory materials, conductive materials, wear-resistant lubrication materials.

Graphite is an allotrope of carbon. It only consists of carbon atoms, so its formula or symbol is C.

Graphite powder is a kind of highly reactive material, which will change its resistivity in according to its environment. But one thing will never change that is the graphite powder is one of the very good non-metallic conductive material. In the object of the insulation, as long as graphite powder is guaranteed uninterrupted, it also will be powered even as a thin line. However, the resistance value of graphite powder can not be told clearly, because the different degree of thickness of graphite powder will cause different applications in different materials and will bring the different resistivity in various environments. Due to its special structure, graphite has the following special properties:

2) Electrical and thermal conductivity: the electrical conductivity of graphite is 100 times higher than that of general non-metallic minerals. Its thermal conductivity is higher than steel, iron, lead and other metal materials. The thermal conductivity decreases with the increase of temperature. Even at extremely high temperature, graphite becomes an adiabatic.
3) Lubricity: the lubrication performance of graphite depends on the size of graphite scale. The larger the scale, the smaller the friction coefficient and the better the lubrication performance.
4) Chemical stability: graphite has good chemical stability at room temperature and can resist acid, alkali and corrosion of organic solvents.
5) Plasticity: graphite has good toughness and can be connected into very thin sheets.
6) Thermal shock resistance: when used at room temperature, graphite can withstand severe changes in temperature without damage. When the temperature changes abruptly, the volume of graphite does not change much and no cracks will occur.
What can the graphite be used for?

2, Graphite can be used as conductive materials: used in the electrical industry as a manufacturing electrode, brush, carbon rod, carbon tube, mercury positive current of the positive pole, graphite gasket, telephone parts, television picture tube coating.
3, Graphite can be used as wear-resisting lubrication material: graphite in the mechanical industry is often used as a lubricant. Lubricating oil can not be used in high speed, high temperature and high pressure conditions, and graphite wear-resisting material can be in (a) 200 ~ 2000 ℃ temperature under high sliding velocity, no lubricating oil. Many conveying corrosive medium equipment use graphite material made of piston cup, seal ring and bearing, and they do not need to add lubricating oil when running. Graphite emulsion is also a good lubricant for many metal processing (wire drawing, tube drawing).

We know that graphite leaves traces on paper only because of the friction between the graphite tip and the surface of the paper. This ensures that the surface of the paper is rough, otherwise the smooth surface of the paper will not allow the graphite molecules to hold their position on the paper, and the molecules will slide.
Due to the irregularity and roughness of graphite surface, the continuity of graphite molecules becomes uneven, there will be no proper flow of electrons, which will make electrical conduction almost impossible.
Because of the roughness of the surface, resistance rises rapidly, impeding the conduction of electricity, the result can be paper burning in high resistance areas.
These irregularities will cause the electrons to move from one point to another with a high potential. As a result, this can lead to paper burning.

TRUNNANO (Luoyang Trunnano Tech Co., Ltd ) is a professional Graphite manufacturer with over 12 years experience in chemical products research and development. If you are looking for high quality Graphite, please feel free to contact us and send an inquiry.
Leave a Reply
Your email address will not be published.Required fields are marked. *
POPULAR BLOG
- What is Cadmium telluride CdTe and CdTe solar cell?
- Four types of surfactants and their differences and applications
- What is the explosive welding cladding of metal plates?
- What is EBS Ethylene Bis Stearamide?
- An article lets you understand the characteristics and applications of sodium silicate
- Advantages of graphite anode for lithium-ion battery
- What is Spherical Quartz Powder?
- What is sodium silicate?
- Characteristics and Application of Spherical Alumina
- Application of Manganese Dioxide
CATEGORIES
